BIOMIMESYS®
New generation of mimetic hydroscaffold for 3D cell culture
Product Basics
The Ideal 3D Cell Culture Model
2D cell culture is not a true reflection of the physiological cell environment. In a real body, cells grow in 3D, connecting to other cells and the extracellular matrix (ECM) to build tissues and organs. Therefore, 3D cell culture can better mimic the original tissue’s specific characteristics. Some of the processes studied in 2D culture such as gene expression, apoptosis, and, importantly, drug uptake and toxicity may not be directly transferable to in vivo experiments.
BIOMIMESYS® is a Hyaluronic acid (HA) based scaffold 3D cell culture model. This groundbreaking 3D cell culture technology associates the behavior of a solid scaffold and of a hydrogel, which we call “Hydroscaffold”. The Hydroscaffold along with its patented technology can mimic different cell’s extra-cellular matrix (ECM).
Key Features
- Composition and mechanical properties are similar to cell’s natural ECM
- Customizable (composition and mechanical properties)
- Long term cell culture (at least 28 days)
- Co-Culture
- Easy and ready to use
- Automation Ready (available in 96 well & 384 well plates)
- Ideal for high throughput screening (HTS) with 3D fluorescence imaging
- Applicable for most downstream applications
- Batch to Batch consistent
Technical Information
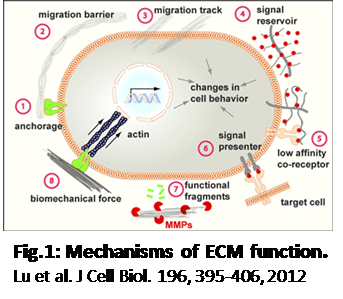
The Importance of Extra-Cellular Matrix (ECM)
The ECM’s important functions are:
- Maintain the structure of tissues and organs
- Define microenvironment for cells
- Determine cell behavior and function
These functions allow cells to anchor and migrate, communicate with each other, and differentiate and proliferate. ECM composition is organ specific. It also dynamically changes through development and function (healthy – diseased).
Side Note: Components of ECM
Structural Elements
- Glycosaminoglycans(GAG)
- Hyaluronic Acid
- Maintains the structure of the tisse
- Blinds to different growth factors and absorb water
- Key to cell-matrix interaction
- Proteoglycans
- Hyaluronic Acid
- Structural Proteins
- Collagen
- Elastin
- Fibronectin
- Laminin
- These proteins, such as nidogen and entactin, are the “glue” that hold the ECM together
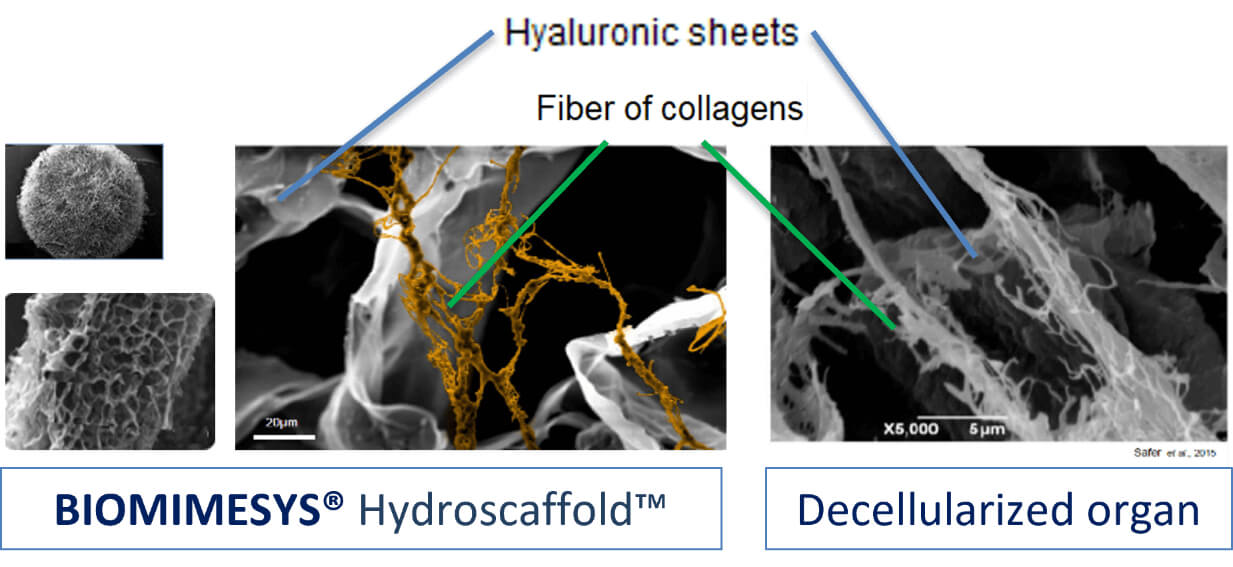
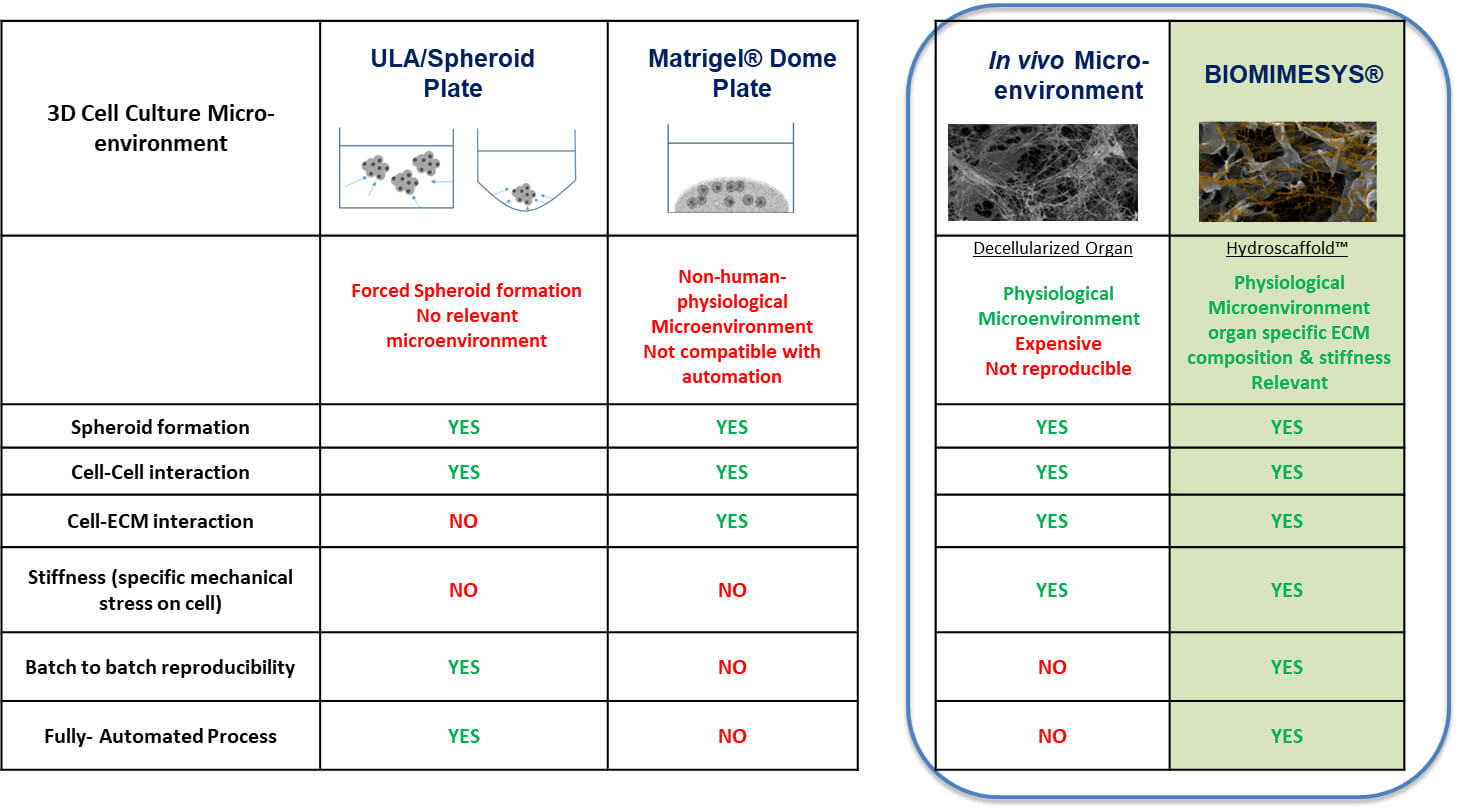
BIOMIMESYS® the Best Alternative
The ideal ECM for research is decellularized organs, but the cost, reproducibility, and scalability make it impractical for continuous use. The following characteristics of the ECM that BIOMIMESYS® provides is the best alternative.
1. Composition
- Hyaluronic acid grafted with adhesion proteins and collagens, elements naturally present in ECM
- Possesses a similar structure in hydrated stage to natural organs
2. Mechanical Properties
- Porosity
- The porous nature of the scaffold provides greater diffusion of oxygen and nutrients for better viability and greatly decreases the formation of necrotic core, often seen in other spherical 3D models.
- The abundance of metabolic enzyme activities and normal cellular polarity makes it ideal for in vitro models
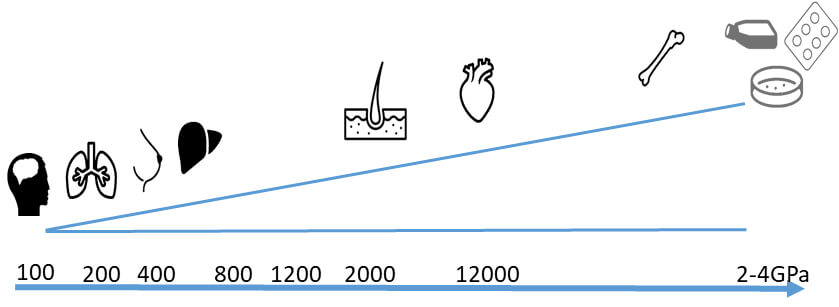
- Mechanical stress (stiffness)
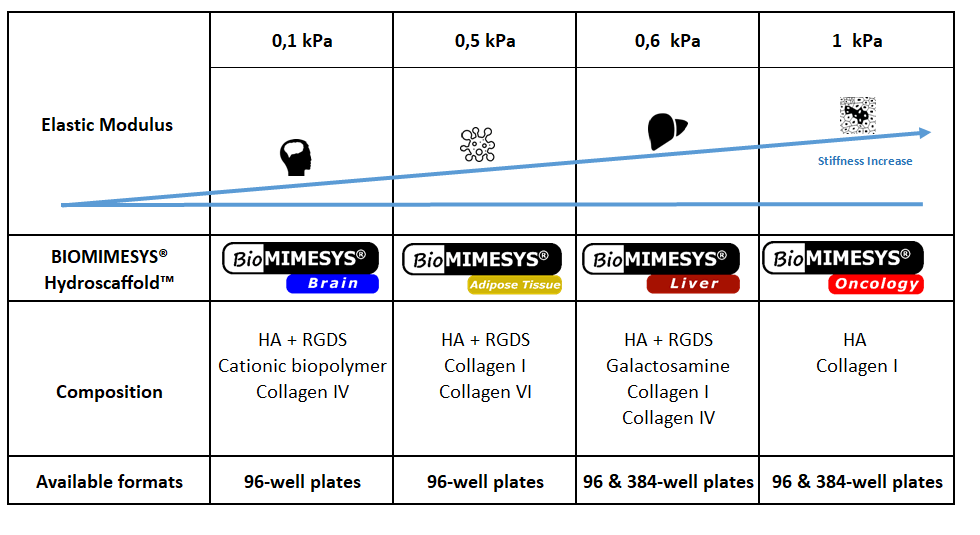
- The stiffness of the ECM differs from organ to organ, furthermore, differs from healthy to diseased.
- BIOMIMESYS® scaffold can be customized to have an elastic modulus of up to 20kPa, whereas with Matrigel the maximum is roughly 0.9kPa.
3. Long Term Viability
The long-term viability of cells is an important factor in cell culture, especially in drug discovery. Cells cultured with BIOMIMESYS® has been tested to have a viable for up to 28 days.
Proof of concept: Increased Culture Time [PDF]
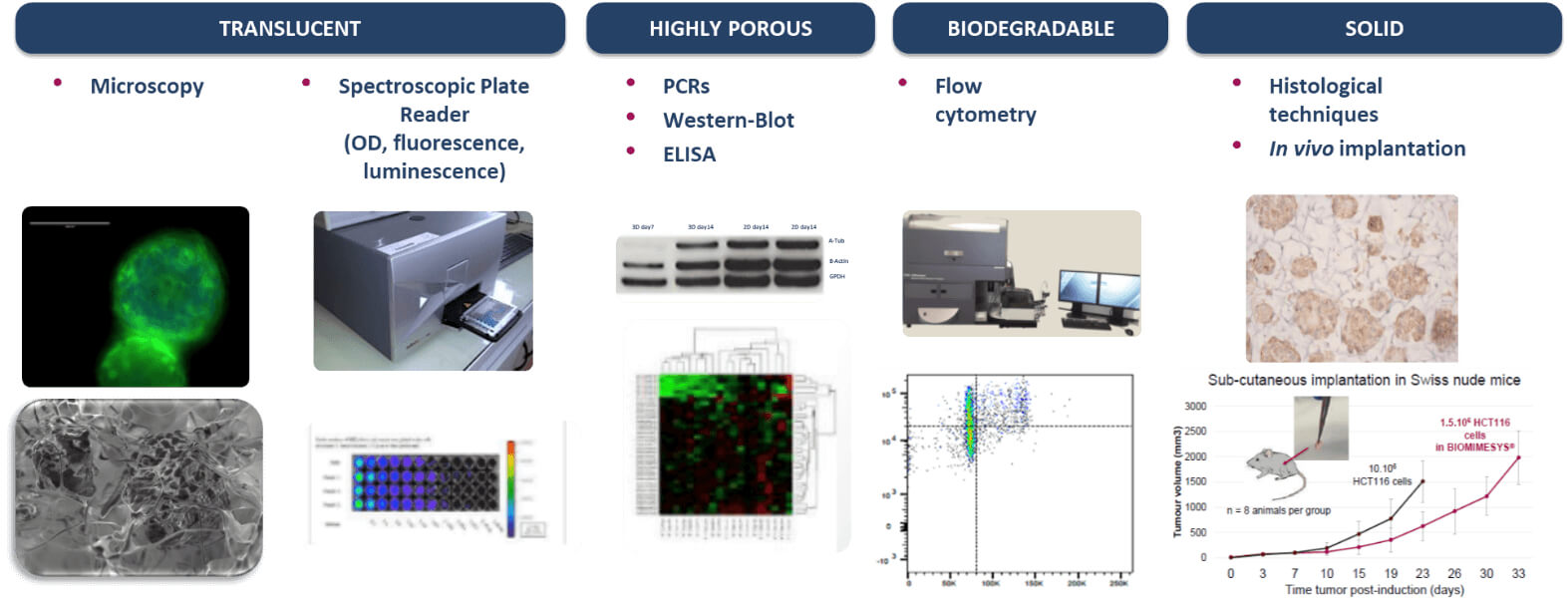
4. Compatible With Most Downstream Applications
BIOMIMESYS® hydroscaffold has many properties (transparent, porous, biodegradable, & solid) making it ideal for use with numerous downstream applications.
5. Ready to Use
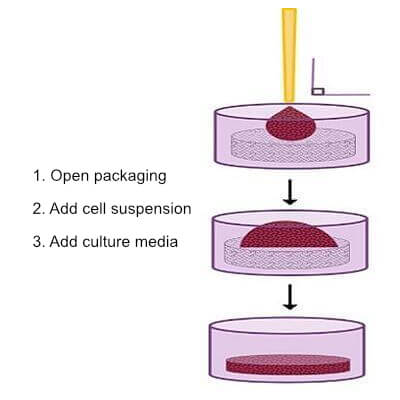
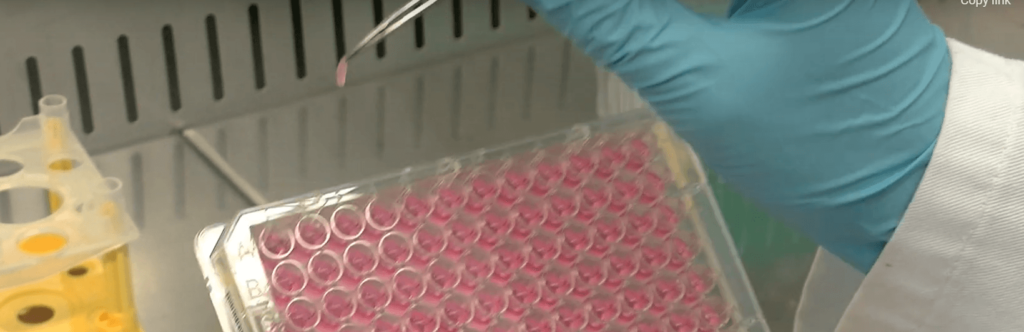
Unlike other models, such as Matrigel, BIOMIMESYS® is ready to use and requires minimal prep time. There is no thawing process, aliquoting, mixing, etc. Just open the packaging, add cell suspension, and add culture media.
Ready-to-use for immediate cell seeding. No preparation required.
Long prep time equals to increase chance of errors,
and waste of resource and time leading to increase in running cost.
Comparison with other models
What does the BIOMIMESYS® Hydroscaffold look like?
Ready to use models
** BIOMIMESYS® composition and mechanical properties (stiffness and porosity) is customizable**
BIOMIMESYS® Series
Related Resources
- Iwai North America Inc.
- Webinars
BIOMIMESYS® Liver Webinar
Applications for 3D in vitro models with BIOMIMESYS® Liver 3D cell culture systems have recently emerged as promising tools for reproducing the cellular environment and the organization of tissues/organs, where cells are connected to each other and to the surrounding extracellular matrix (ECM). In this webinar we will focus on BIOMIMESYS® Liver and will discuss […]
- Iwai North America Inc.
- News
BIOMIMESYS® 3D cell culture scaffold as powerful tool to study metastases of breast cancer
Exciting news! A groundbreaking study using BIOMIMESYS® for breast cancer metastasis research has been published in the Experimental Hematology & Oncology Journal. The study, titled “ProNGF promotes brain metastasis through TrkA/EphA2 induced Src activation in triple negative breast cancer cells” by Cicero, Trouvilliez, et al. (2023), emphasizes the role of Extracellular Matrix organ specificity in […]
- Iwai North America Inc.
- Webinars
BIOMIMESYS® Adipose Tissue Webinar
BIOMIMESYS® hydroscaffold technology based on crosslinking Hyaluronic Acid (HA) and extracellular matrix compounds reproduces tissue microenvironment in 3D. In this webinar we will focus on BIOMIMESYS® Adipose Tissue and its applications. Due to the tunable mechanical and chemical properties, this matrix possess biophysical characteristics similar to natural tissue and represent highly effective matrice for the […]
- Iwai North America Inc.
- Webinars
BIOMIMESYS® Webinar
How to create physiologically-relevant 3D in vitro models using BIOMIMESYS® hydroscaffold BIOMIMESYS® is a Hyaluronic acid (HA) based scaffold 3D cell culture model. This groundbreaking 3D cell culture technology associates the behavior of a solid scaffold and of a hydrogel, which we “Hydroscaffold”. This Hydroscaffold along with its patented technology can biomimic different cell’s extra-cellular […]
References
1.Lu, P., Weaver, V. M. & Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J Cell Biol 196, 395 (2012).
2.Kola, I. & Landis, J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov 3, 711–715 (2004).
3.Wang, L., Johnson, J. A., Zhang, Q. & Beahm, E. K. Combining decellularized human adipose tissue extracellular matrix and adipose-derived stem cells for adipose tissue engineering. Acta Biomater 9, 8921–8931 (2013).Mazza, 2015
4.Mazza, G. et al. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci Rep 5, 13079 (2015).
5.Lodish, H. et al. Integrating Cells Into Tissues. in Molecular Cell Biology (Freeman, W. H. & Company, 2003).
6.Peach, R. J., Hollenbaugh, D., Stamenkovic, I. & Aruffo, A. Identification of hyaluronic acid binding sites in the extracellular domain of CD44. J Cell Biol 122, 257–264 (1993).
7. Colom, A. et al. Oxygen diffusion and consumption in extracellular matrix gels: implications for designing three-dimensional cultures. J Biomed Mater Res A 102, 2776–2784 (2014).
8.McMurtrey, R. J. Analytic Models of Oxygen and Nutrient Diffusion, Metabolism Dynamics, and Architecture Optimization in Three-Dimensional Tissue Constructs with Applications and Insights in Cerebral Organoids. Tissue engineering. Part C, Methods 22, 221–49 (2016).
9. Al-Ani, A. et al. Oxygenation in cell culture: Critical parameters for reproducibility are routinely not reported. PLoS One 13, e0204269 (2018)
FOR RESEARCH USE ONLY, NOT FOR USE IN DIAGNOSTIC PROCEDURES