HSC-BANKER® GMP grade
Preserving cells for regenerative medicine and cell therapy research
Product Basics
HSC-BANKER® GMP Grade is an optimized cryopreservation medium for hematopoietic stem cells. It is a ready-to-use product, and reports indicate that the cryopreservation outcome of HSC-Banker is at least equivalent to the conventional protocol using DMSO and DEXTRAN.
※ The composition of this product is identical to that of STEM-CELLBANKER® EX GMP grade. We recommend using STEM-CELLBANKER® EX GMP grade when a blood freezing bag is not used.
Key Features
- Free of serum and animal derived component
- Useful for the cryopreservation of human hematopoietic stem cells and human adipose-derived stromal/stem cells
- Batch-to-batch stability
- A high-quality product that is carefully monitored in compliance with GMP
* This product is U.S. FDA DMF registered. Contact us to request Letter of Authorization submission.
Technical Information
Comparative study
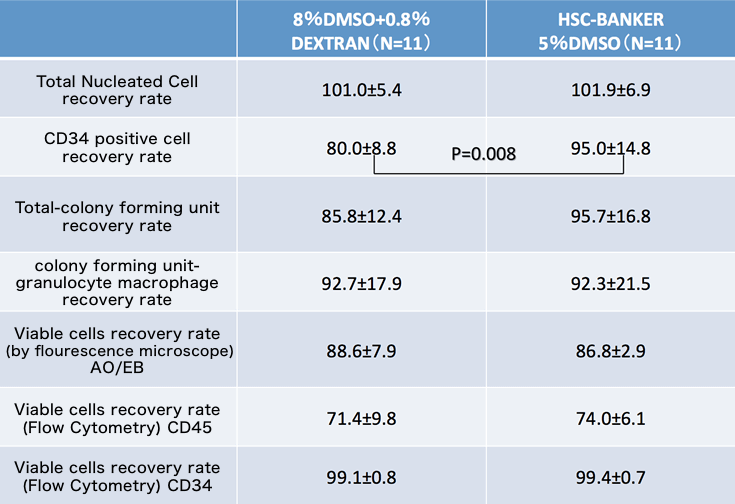
The results of a comparative study on the cryopreservation of the hematopoietic stem cells performed the Cord Blood Bank of the Japanese Red Cross Society. The following data was provided by Japan Red Cross Society
The Japan society for Hematopoietic cell transplantation (2015)
Cells Tested:
| Cell Type | Products | Contributor |
|---|---|---|
| Adipose-Derived Stromal/Stem Cells (ADSC) | STEM-CELLBANKER® EX GMP grade | EMO Biomedicine |
| Cord blood derived hematopoietic stem cells | HSC-BANKER® GMP grade | The Japan society for Hematopoietic cell transplantation (2015) |
Protocol
Freezing protocol – using a cord blood freezing bag (HSC-BANKER® GMP grade)
NOTE: The composition of HSC-BANKER® GMP grade is the same as STEM-CELLBANKER® EX GMP grade. We recommend using STEM-CELLBANKER® EX GMP grade when blood freezing bag is not used.
- Remove red blood cells from the cord blood collection.
- Separate the cord blood into plasma and buffy coat fractions by centrifugation at 400 x g for 10 minutes.
- Reduce the volume of the cord blood to 13 mL by removing excess plasma.
- Gently add equal volume (13 mL) of HSC-BANKER® GMP grade to the cord blood. Place the freezing bag in a controlled rate freezer to gradually freeze the cord blood to -80°C.
- After the cord blood has reached the temperature of below -80°C, transfer it to a liquid nitrogen tank for long term storage.
Specification
- Manufactured By : Zenogen Pharma Co., Ltd
- Size : 15mL
- Storage temperature : 2 to 8℃
- Expiration date : 3 years after the date of manufacture (see label)
- Disclaimer: HSC-BANKER® GMP grade is not itself a pharmaceutical. Therefore, no warranty, express or implied, as to the fitness and suitability of this product for any particular purpose and/or merchantability unless the use is intended for research.
Pricing
HSC-BANKER® GMP grade
- Designed for HSC
- iPS・ES cells cryopreservation
- Using ingredients for intravenous injection
- Animal component free・Protein free
- Size: 15mL
- Price: $50.00
* This product is U.S. FDA DMF registered. Contact us to request Letter of Authorization submission.
References & Literature
Mizui, T., Chen, H., Lee, S. & Fukui, M. Study on cryopreservation of human adipose-derived stromal cells. Cytotherapy 23, S60–S61 (2021) doi: 10.1016/S1465324921003546.
FOR RESEARCH USE ONLY, NOT FOR USE IN DIAGNOSTIC PROCEDURES