HemEx™-Type9AØ
Mouse HSC expansion basal medium
Product Basics
HemEx™ – Type9AØ, the new improved version of the HemEx™ – Type9A, is a basal medium specifically designed for the expansion of mouse hematopoietic stem cells (HSCs) isolated from bone marrow. Contaminants derived from albumin can trigger the differentiation of HSCs. By substituting albumin with Soluplus®, HemEx™ – Type9AØ can maintain HSCs in an undifferentiated state for over one month during expansion culture.
Key Features
- Serum-free formulation
- Verified more proliferation of mouse hematopoietic stem (HSCs) and progenitor cells (HSPCs) than conventional products
- FACS purification not necessary
- Developed under the supervision of Prof. Hiromitsu Nakauchi (Stanford University) and Prof. Satoshi Yamazaki (Tokyo University), based on the Wilkinson et al. 2019 publication
Technical Information
Comparison between HemEx™-Type9A vs HemEx™-Type9AØ
Scroll to right
| Product Name Catalog# |
HemEx™-Type9AØ (New) A5P10P01C |
HemEx™-Type9A (Discontinued) A5P00P01C |
|---|---|---|
| Albumin Substitute | Soluplus® | PVA (polyvinyl alcohol) |
| Proliferation of undifferentiated HSC | Increased* | Higher compared to conventional media |
| Expansion from single HSC | Yes | No |
| Ratio of HSC to HSPC during culture | Higher (maintains more CD201+/CD150+)* | Higher compared to conventional media |
Scroll to right
* Becker et al. 2023
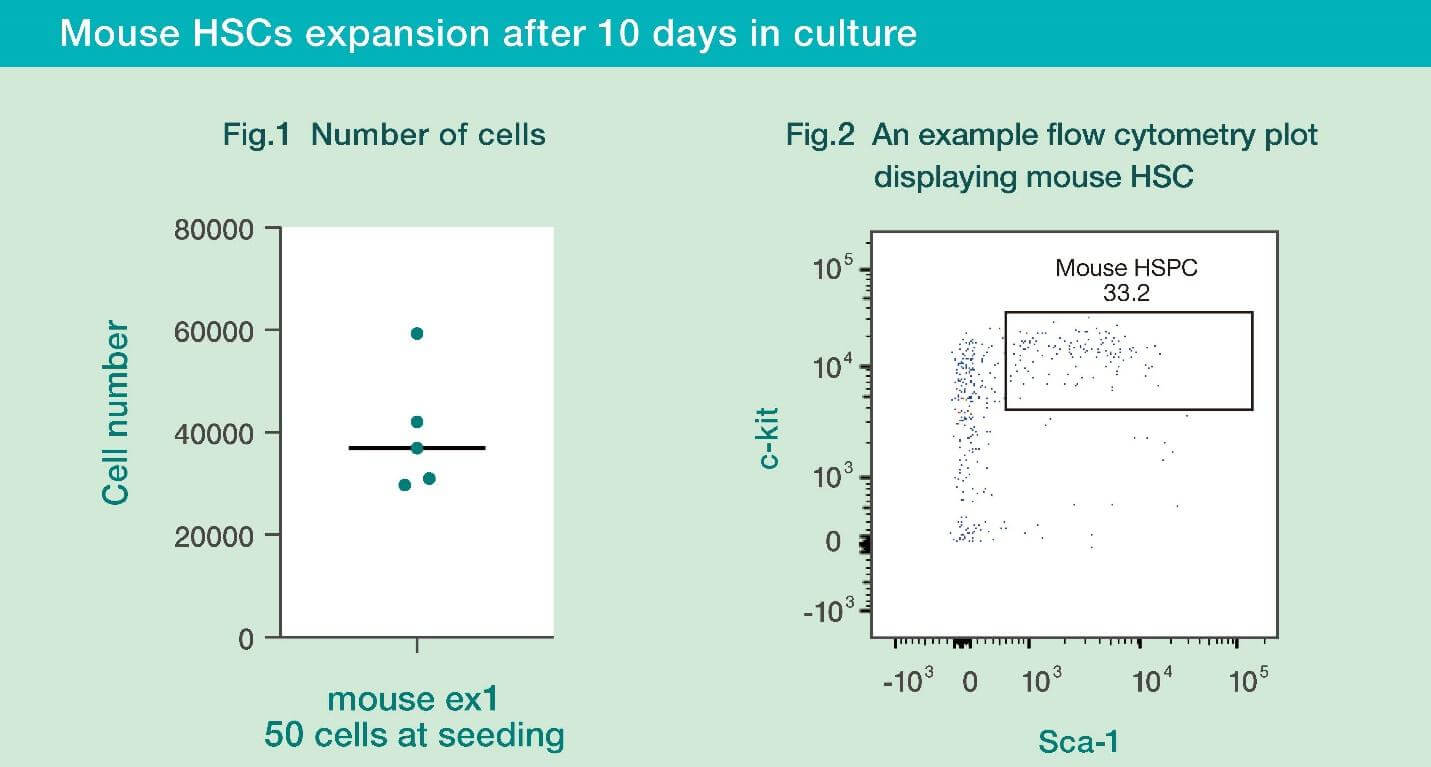
- Mouse HSCs cultured with HemEx™-Type9AØ proliferated well for 10 days.
- When the cultured mouse HSC cells were analyzed by flow cytometry, it was confirmed that the cultured cells maintained their stemness.
*Requires the addition of cytokines
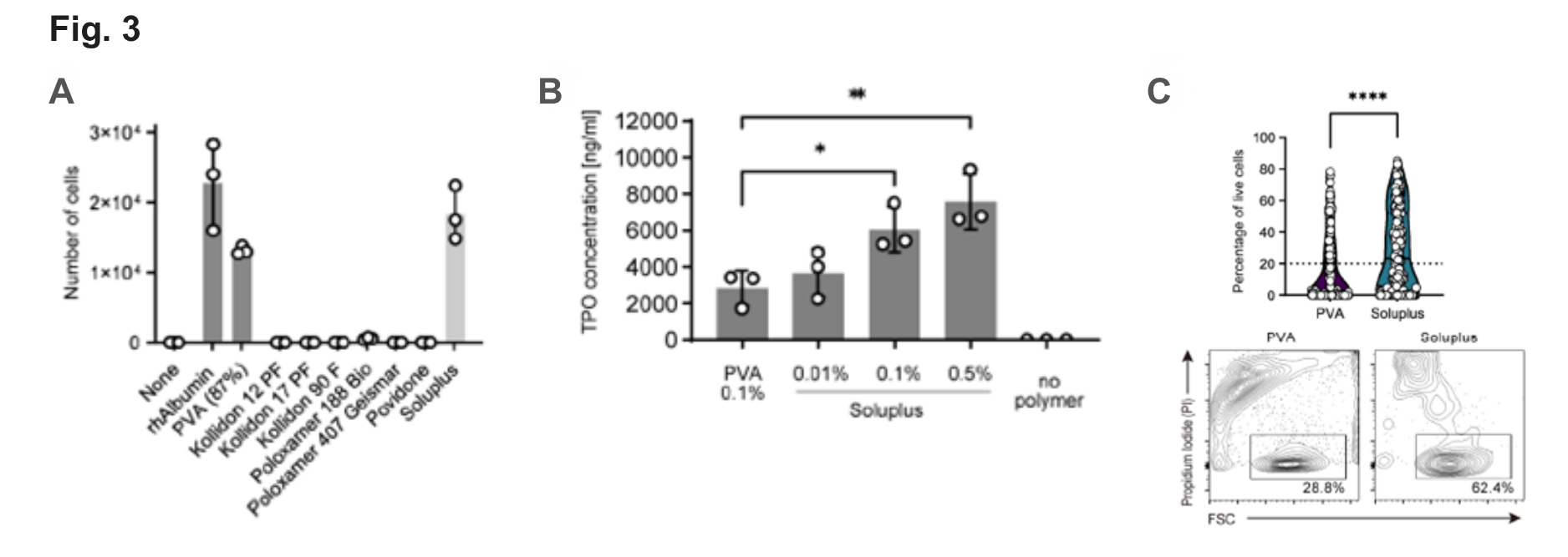
Fig. 3
(A) Expansion of murine HSCs for 7 days with various albumin replacement polymer.
(B) Murine TPO ELISA. Ham’s F12 with 100 ng/ml Thrombopoietin (TPO) was supplemented with the indicated polymers and cultured for 3 days. TPO levels were significantly higher if Soluplus is present compared with plain or PVA-supplemented medium.
(C) Percentage of viable cells in HSC colonies grown from freshly isolated single CD34-CD150+KSL cells after 19 days of culture in PVA (n=288)- and Soluplus (n=290)-based media (flow cytometry ). Representative FACS plots of single-cell derived colonies (day 19).
Specification
- Size: 100mL (bottle)
- Storage temperature: 2-8°C
- Shelf life: 1 year following manufacture date
- Manufactured by: Cell Science and Technology Institute
Pricing
References
- Wilkinson, A. C. et al. Long-term ex vivo haematopoietic-stem-cell expansion allows nonconditioned transplantation. Nature 571, 117–121 (2019) doi: 1038/s41586-019-1244-x.
- Sato, R. et al. The impact of cell maturation and tissue microenvironments on the expression of endosomal Toll-like receptors in monocytes and macrophages. International Immunology 32, 785–798 (2020) doi: 1093/intimm/dxaa055.
- Wilkinson, A. C., Ishida, R., Nakauchi, H. & Yamazaki, S. Long-term ex vivo expansion of mouse hematopoietic stem cells. Nature Protocols 15, 628–648 (2020) doi: 1038/s41596-019-0263-2.
- Ochi, Kiyosumi, Morita, Maiko, Wilkinson, Adam, Iwama, Atsushi, & Yamazaki, Satoshi. Cell culture-based enrichment of mouse hematopoietic stem and progenitor cells. Protocol Exchange (2021) doi: 21203/rs.3.pex-1481/v1.
- Ochi, K., Morita, M., Wilkinson, A. C., Iwama, A. & Yamazaki, S. Non-conditioned bone marrow chimeric mouse generation using culture-based enrichment of hematopoietic stem and progenitor cells. Nature Communications 12, 3568 (2021) doi: 1038/s41467-021-23763-z.
- Shiroshita, K. et al. A culture platform to study quiescent hematopoietic stem cells following genome editing. Cell Reports Methods 2, 100354 (2022) doi: 1016/j.crmeth.2022.100354.
- Becker, H. J. et al. Controlling genetic heterogeneity in gene-edited hematopoietic stem cells by single-cell expansion. Cell Stem Cell 30, 987-1000.e8 (2023) doi: 10.1016/j.stem.2023.06.002.
- Igarashi, K. J. et al. Physioxia improves the selectivity of hematopoietic stem cell expansion cultures. Blood Advances 7, 3366–3377 (2023) doi: 1182/bloodadvances.2023009668.
- Omata, Y., Tachibana, H., Aizaki, Y., Mimura, T. & Sato, K. Essentiality of Nfatc1 short isoform in osteoclast differentiation and its self-regulation. Scientific Reports 13, 18797 (2023) doi: 10.1038/s41598-023-45909-3.
- Kawano, H. et al. Mitochondrial Transfer to Host Cells from Ex Vivo Expanded Donor Hematopoietic Stem Cells. Cells 12, (2023) doi: 3390/cells12111473.
- Skinder, N., Fernandez, I. S., Dethmers-Ausema, A., Weersing, E. & de Haan, G. CD61 identifies a superior population of aged murine HSCs and is required to preserve quiescence and self-renewal. Blood advances 8, 99–111 (2024) doi: 1182/bloodadvances.2023011585.
Other Documents
FOR RESEARCH USE ONLY, NOT FOR USE IN DIAGNOSTIC PROCEDURES